Introduction
Cutaneous T-cell lymphoma (CTCL) is a rare form of non-Hodgkin lymphoma. Sézary syndrome (SS) is a rare, aggressive and advanced type of CTCL. It has a unique biology, with high expression of KIR3DL2, a killer immunoglobulin-like receptor, reported in more than 85% of patients. SS is characterized by erythroderma, significant blood involvement with malignant SS cells, and lymphadenopathy. These patients suffer from debilitating itching and recurrent skin infections, often affecting quality of life (QoL). SS is distinguished by its poor prognosis, as the median survival of patients is approximately 5 years. Lacutamab is a first-in-class monoclonal antibody designed to specifically deplete KIR3DL2-expressing cells via antibody-dependent cell-cytotoxicity and phagocytosis.
Methods
TELLOMAK is an international, open-label, Phase 2 trial with multiple cohorts (NCT03902184). We report here results from Cohort 1, designed to evaluate safety and efficacy of single agent lacutamab in patients with relapsed/refractory (R/R) SS after at least 2 prior systemic therapies including mogamulizumab. Patients had blood stage B2 (≥1000 circulating Sézary cells/mm 3) at screening based on central evaluation by flow cytometry. Patients with evidence of large cell transformation were excluded. Lacutamab 750 mg is administered as an intravenous infusion weekly × 5 weeks (w), every 2 w × 10, then every 4 w until progression or unacceptable toxicity. Primary endpoint was Objective Response Rate (ORR) by global response score based on the evaluation of 4 compartments: skin, blood, lymph nodes and viscera according to International Consensus criteria (Olsen 2011). Secondary endpoints included but not limited to additional efficacy endpoints, safety, QoL assessments.
Results
At the data cut-off of May 1, 2023, 56 SS patients were enrolled treated and evaluated in Cohort 1. Median age was 69 years (range: 42-86), the median prior lines of systemic therapies was 6.0 (range: 2-15), 60.7 % (n=34) had stage IVA1, 32.1 % (n=18) had stage IVA2 and 7.1% (n=4) had stage IVB disease at baseline, all patients (100%) had blood involvement (B2), 67.9% (n=38) had confluence of erythema covering ≥ 80% body surface area (T4), 35.7% (n=20) had lymph node lymphoma involvement (N3). Median follow-up was 14.4 months (95% CI 9.0-18.4).
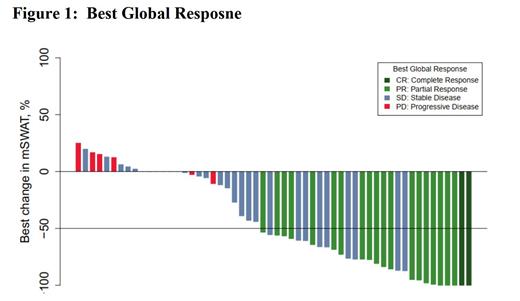
In the ITT population (n=56), Global confirmed ORR was 37.5% (n=21; 95% CI 26.0-50.6) including 2 CRs, with a median time to response of 2.8 months (range 1-9; Figure 1). Confirmed ORR in skin was 46.4% (n=26; 95% CI 34.0-59.3) including 5 CRs. Confirmed ORR in blood was 48.2% (n=27; 95% CI 35.7-61.0) including 15 CRs. Clinical Benefit Rate (CBR, defined as CR+PR+SD) was 87.5 % (n=49; 95% CI 76.4-93.8). Median PFS was 8.0 months (95% CI 4.7-21.2). Grade ≥ 3 Treatment-related (TR) Treatment-Emergent Adverse events (TEAEs) were observed in 10/56 (17.9%) pts, Serious TR TEAEs were observed in 4/56 (7.1%) and 3/56 (5.4%) pts discontinued study drug due to TR TEAE. Data from additional key endpoints will be presented.
Conclusion
In this SS cohort from the TELLOMAK study, our data confirm that lacutamab monotherapy shows promising clinical activity in a R/R population previously treated with 2 or more prior systemic therapies including mogamulizumab, and an overall favourable safety profile. Continued evaluation of this new targeted treatment option for patients with SS is warranted.
Disclosures
Kim:Innate: Research Funding; Kyowa Kirin: Research Funding; Trillium: Research Funding; Elorac: Research Funding; CRISPR Therapeutics: Research Funding; Takeda: Research Funding; Corvus: Research Funding; Eisai: Research Funding; Citius: Research Funding; Drenbio: Research Funding. Ortiz-Romero:Therakos: Consultancy; Mallinckrodt: Consultancy; Innate Pharma: Consultancy; 4SC: Consultancy; Kyowa Kirin: Consultancy; Helsinn: Consultancy; Recordati rare diseases: Consultancy. Mehta-Shah:Corvus Pharmaceuticals: Research Funding; Janssen: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Kyowa Hakko: Consultancy; Genentech: Consultancy; AstraZeneca: Consultancy, Research Funding; Bristol Myers-Squibb: Research Funding; Celgene: Research Funding; Ono Pharmaceuticals: Consultancy; Genentech/Roche: Research Funding; Secura Bio/Verastem: Consultancy, Research Funding; C4 Therapeutics: Consultancy, Research Funding; Innate Pharmaceuticals: Research Funding; Karyopharm Therapeutics: Consultancy. Jacobsen:Pharmacyclics: Research Funding; Merck: Honoraria, Research Funding; Celgene: Research Funding; UpToDate: Patents & Royalties; BMS: Honoraria; Bayer: Honoraria; Hoffman-LaRoche: Research Funding; Daiichi: Honoraria. Khodadoust:Nutcracker Therapeutics: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Research Funding. Battistella:Innate Pharma: Consultancy; Takeda: Consultancy; Kyowa Kirin: Research Funding; Bristol Myers: Consultancy. Gru:Innate Pharma: Consultancy. Moins-Teisserenc:Innate Pharma: Consultancy. Zinzani:JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Viotti:Innate Pharma: Current Employment. Paiva:Innate Pharma: Current Employment. Muller:Innate Pharma: Current Employment. Porcu:Kymera: Membership on an entity's Board of Directors or advisory committees; Kyowa: Consultancy; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; BioGene: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal